When you eat bread, pasta, or beer, your body doesn’t usually react-unless you have celiac disease. This isn’t just a food sensitivity or a trendy diet choice. It’s a serious autoimmune disorder where your immune system attacks your own small intestine every time you consume gluten. And it’s more common than you think: about 1 in 100 people worldwide have it, and many don’t even know it yet.
What Happens Inside Your Body When You Eat Gluten?
Celiac disease doesn’t start with a stomachache. It starts with a genetic red flag. If you carry the HLA-DQ2 or HLA-DQ8 genes, your body is wired to react dangerously to gluten-a protein found in wheat, barley, and rye. When gluten enters your digestive tract, it doesn’t break down fully. Instead, stubborn fragments like the 33-mer gliadin peptide slip through the gut lining. This isn’t normal. Healthy intestines keep these particles out. But in celiac disease, the gut barrier gets leaky, thanks to a protein called zonulin that opens the gaps between cells.
Once those gluten fragments slip into the tissue beneath the gut lining, they meet an enzyme called tissue transglutaminase 2 (TG2). TG2 chemically alters them, making them look like a threat to your immune system. Your body then sees these altered gluten pieces as invaders. Special immune cells called CD4+ T cells get activated and start firing off inflammatory signals like interferon-gamma and interleukin-21. These chemicals don’t just cause inflammation-they destroy the finger-like projections in your small intestine called villi. That’s the damage that leads to malnutrition, fatigue, and long-term complications.
Here’s the twist: new research from McMaster University in August 2024 showed that your gut lining isn’t just a passive victim. Cells in the intestinal epithelium can actually start the immune attack themselves if they express the same HLA molecules linked to celiac disease. This flips the old idea that only immune cells trigger the response. Now we know your gut cells are active players in the disease process.
Celiac Disease vs. Gluten Sensitivity: What’s the Real Difference?
Not every reaction to gluten is celiac disease. Many people feel better cutting out wheat, but they don’t have the same immune response. This is called non-celiac gluten sensitivity (NCGS). The numbers tell the story: celiac affects about 1% of the population, while NCGS could affect up to 13%-but it’s harder to pin down.
The key differences are clear:
- Immune markers: Celiac patients have specific antibodies like anti-tTG (tissue transglutaminase), which show up in blood tests with 98% accuracy. NCGS has no reliable blood test.
- Intestinal damage: Celiac causes visible destruction of the villi-confirmed by biopsy. NCGS shows no such damage.
- Genetics: Only people with HLA-DQ2 or HLA-DQ8 genes can develop celiac. NCGS has no known genetic link.
- Dietary tolerance: People with NCGS might handle small amounts of gluten without serious harm. For someone with celiac, even a crumb can cause damage.
And here’s the catch: many people self-diagnose as gluten-sensitive without testing. That’s risky. If you cut out gluten before getting tested, you might hide celiac disease from blood tests and biopsies. Doctors can’t diagnose it if you’re not eating gluten. Always get tested first.
How Is Celiac Disease Diagnosed?
Diagnosis isn’t a single test. It’s a chain of evidence. First, your doctor will order a blood test for anti-tTG-IgA antibodies. This test is highly accurate-98% sensitive and 95% specific-if you’re still eating gluten. If it’s positive, the next step is an endoscopy. A thin tube with a camera is passed down your throat to take tiny tissue samples from your small intestine. Pathologists look for Marsh classification scores: Grade 3 means severe villous atrophy, the hallmark of celiac disease.
Genetic testing for HLA-DQ2/DQ8 is also used-but not to diagnose. It’s used to rule out. If you don’t have these genes, you almost certainly don’t have celiac. That’s 99% accurate. So if your blood test is positive but your genes are negative, something else is going on.
And timing matters. You must be eating gluten regularly for at least 6 weeks before testing. Going gluten-free too early can give you a false negative. That’s why so many people wait years for a diagnosis. The Celiac Disease Foundation found that the average delay is 6.7 years. One Reddit user waited seven years and saw four different doctors before getting a biopsy that confirmed it.

Why the Gluten-Free Diet Isn’t Just a Trend-It’s Lifesaving
There’s no pill, no shot, no cure. The only treatment right now is a strict, lifelong gluten-free diet. That means no bread, no pasta, no beer, no soy sauce unless it’s labeled gluten-free. Even trace amounts matter. The international standard allows up to 20 parts per million (ppm) of gluten in products labeled “gluten-free.” That’s tiny-like one drop in a swimming pool-but for someone with celiac, even that can trigger an immune response.
Getting started isn’t easy. You’ll need to:
- Empty your pantry and replace anything with wheat, barley, or rye
- Learn to read labels for hidden gluten: malt, hydrolyzed vegetable protein, modified food starch, dextrin, and even some flavorings
- Use separate kitchen tools: a dedicated toaster, cutting board, and utensils reduce cross-contamination by 85%
- Carry gluten-free snacks when you’re out: 92% of experienced patients do this
- Use apps like Find Me Gluten Free to locate safe restaurants (rated 4.7 stars by 185,000 users)
Cost is a real barrier. Gluten-free products cost 242% more on average than regular ones, according to Consumer Reports in 2023. But the payoff is huge. In a survey of over 15,000 people, 89% reported major improvement within six months of going gluten-free. Fatigue fades. Digestion improves. Brain fog lifts.
What No One Tells You About Living Gluten-Free
It’s not just about food. It’s about your whole life.
Medications are a hidden danger. Only 37% of prescription drugs list gluten content. Pills, capsules, and even some topical creams can contain gluten as a filler. Always check with your pharmacist.
Restaurants are risky. A 2023 survey found that 67% of celiac patients have experienced cross-contamination while dining out-even at places that claim to be “gluten-free friendly.”
And emotionally, it’s tough. 58% of people report moderate to severe stress from dietary restrictions. Social events, family dinners, and holidays become minefields. Young adults often avoid gatherings altogether. But support helps. Online communities like r/celiac (with over 245,000 members) and Beyond Celiac offer real advice, recipes, and validation.
One user shared: “After five years on a strict gluten-free diet, my follow-up endoscopy showed complete healing. But staying under 20 ppm? That’s a full-time job.”
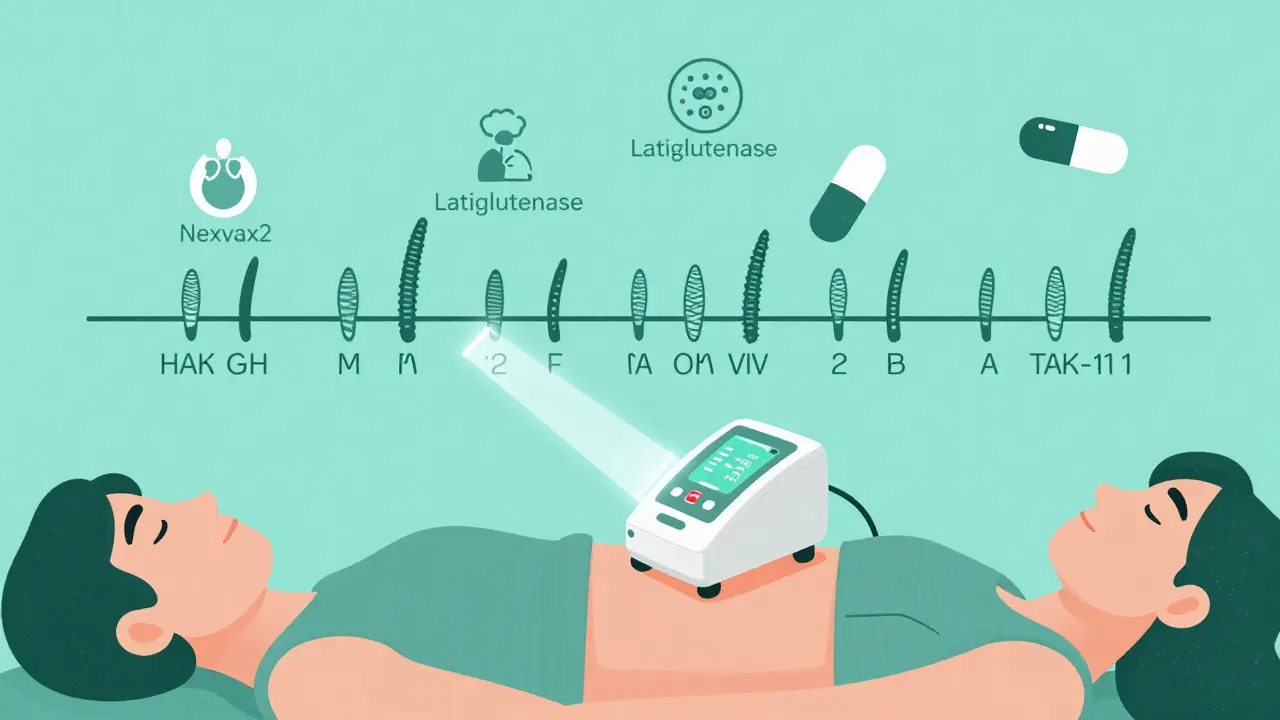
What’s Coming Next? The Future of Celiac Treatment
Scientists aren’t giving up. While the gluten-free diet remains the gold standard, new therapies are in the pipeline:
- Nexvax2: A vaccine-like therapy that desensitizes the immune system to gluten. Phase 2 trials in 2023 showed a 42% reduction in symptoms.
- Latiglutenase: An enzyme that breaks down gluten in the stomach before it reaches the intestine. It improved symptoms by 37% in a 2022 trial.
- TAK-101: A nanoparticle treatment that teaches the immune system to ignore gluten. In a 2023 study, it cut intestinal damage by 63%.
Researchers are also working on tools to detect gluten at home. Early prototypes can spot gluten at just 5 ppm-more sensitive than current lab tests. And there’s growing interest in the gut microbiome: 78% of celiac patients show unique bacterial patterns that might influence disease severity.
But here’s the reality: even with these advances, the gluten-free diet will remain the main treatment through at least 2030. These new therapies won’t replace diet-they’ll support it.
What You Should Do Now
If you’ve had unexplained fatigue, bloating, diarrhea, or anemia for months or years, get tested. Don’t cut out gluten until you’ve been screened. You could be missing a diagnosis that changes everything.
If you’ve already been diagnosed, you’re not alone. Millions are managing this daily. Connect with support groups. Learn your triggers. Track your symptoms. And remember: healing takes time. Villi regrow slowly-sometimes over a year or two. But they do heal.
And if you’re eating gluten-free because you feel better, don’t assume it’s just a trend. For some, it’s the difference between chronic illness and a normal life. For others, it’s a medical necessity.
Celiac disease isn’t about willpower. It’s about biology. And understanding that biology is the first step to living well with it.
Can celiac disease be cured?
No, celiac disease cannot be cured. It’s a lifelong autoimmune condition. The only effective treatment is a strict, lifelong gluten-free diet. While new therapies are in development, none have replaced dietary management yet. Even with promising drugs in trials, avoiding gluten remains essential to prevent intestinal damage and complications.
Can I eat oats if I have celiac disease?
Pure, uncontaminated oats are generally safe for most people with celiac disease. But most commercial oats are processed in facilities that also handle wheat, barley, or rye. Look for oats labeled “certified gluten-free.” Even then, some people react to avenin, a protein in oats similar to gluten. Start with small amounts and monitor symptoms. Always consult your doctor before adding oats to your diet.
Why do I still have symptoms after going gluten-free?
Up to 30% of people with celiac disease continue to have symptoms despite a gluten-free diet. This is often due to accidental gluten exposure-like cross-contamination in kitchens, restaurants, or medications. Other causes include lactose intolerance (common after intestinal damage), small intestinal bacterial overgrowth (SIBO), or other conditions like IBS. If symptoms persist, see a specialist for further testing.
Is celiac disease the same as a wheat allergy?
No. A wheat allergy is an IgE-mediated immune reaction that causes immediate symptoms like hives, swelling, or anaphylaxis. Celiac disease is an autoimmune disorder that causes delayed damage to the small intestine over time. The immune mechanisms, symptoms, and long-term risks are completely different. You can have one, both, or neither.
Do I need to avoid gluten if I only have a family history of celiac disease?
No. If you don’t have symptoms or positive blood tests, you don’t need to avoid gluten. But if you carry the HLA-DQ2 or HLA-DQ8 genes, you’re at higher risk. Monitor for symptoms like chronic diarrhea, weight loss, fatigue, or anemia. Get tested if they appear. Avoiding gluten unnecessarily can make future diagnosis harder and may lead to nutrient deficiencies.
For more information, refer to the Celiac Disease Foundation, the National Institutes of Health, and peer-reviewed journals like Gastroenterology and Frontiers in Immunology.
Comments (11)